Niclosamide is a small molecule with potent and broad-spectrum anti-infective properties, initially in COVID-19 but with broader potential in other respiratory infections. Niclosamide has a host-targeting dual mode-of-action (MoA), which reduces viral replication in cells and helps cells to clear viruses through an increase in autophagic flux. Niclosamide has demonstrated a 40-fold higher in vitro potency against SARS-CoV-2 compared to remdesivir in preclinical studies undertaken during 2020 and has been demonstrated in vitro to be effective against a range of COVID-19 variants.
Niclosamide is a salicylanilide introduced into clinics in the early 1960s as an oral treatment for tapeworm infections and is currently marketed in several European and developing countries. Niclosamide in its oral form has poor systemic availability limiting its use as a treatment for respiratory viruses. Therefore, UNION has developed a concentrated liquid formulation of niclosamide that allows for delivery as a nasal spray to the upper respiratory tract (niclosamide nasal spray). For respiratory infections, such as COVID-19, this allows for a local delivery of niclosamide directly to the site of infection, either as a potential treatment or, prior to infection, as a prophylactic agent.
Mild-to-Moderate
13,4M suffers from Psoriasis in the US, EU5 and Japan
PDE4 inhibitor
Dermatology market and include
topical roflumilast cream
PDE4 Market and include
topical roflumilast cream
Moderate-to-servere
People in the US suffer from Psoriasis
TNF-Q + 11-17
Plaque psoriasis and atopic
dermatitis
Acim quam ratem eos dis ma
doluptatist
Checkpoint-for Psoriasis
People in EU5 suffer from Psoriasis
Keratinoc
Exerting anti-inflammatory effect
reducing psoriasis scale formation
PDE4 Market and include
topical roflumilast cream
Niclosamide development programs
UNION completed Phase 1 testing in healthy volunteers (ClinicalTrials.gov Identifier: NCT04576312) during 2020 with niclosamide nasal spray. The nasal spray was selected for the COVID-19 platform study (PROTECT-V) (ClinicalTrials.gov Identifier: NCT04870333) and topline results were reported in June 2023. The study did not meet its primary endpoint, as no difference was detected between risk of infection in the niclosamide and placebo groups. No major safety signals were reported in the study.

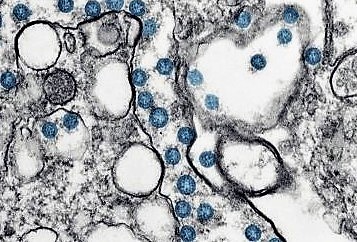
COVID-19
COVID-19 is a disease caused by infection with SARS-CoV-2, a strain of coronavirus. Respiratory illness is the most common symptom associated with COVID-19 with a severity ranging from mild disease to life-threatening acute respiratory distress syndrome. Patients with advanced age, comorbidities such as obesity, diabetes and cardiovascular disease, or an immunocompromised state, are at increased risk for poor outcomes. COVID-19 was declared a pandemic by the World Health Organization (“WHO”) in 2020. According to the WHO, as of August 2, 2022, more than 572 million people have been infected and COVID-19 has caused more than 6.3 million deaths.
COVID-19 infects humans via the mouth and nasal cavity where it replicates and then progresses into the airways and lungs. The most common symptoms of COVID-19 are fever, dry cough and fatigue, and less common symptoms include soreness of body and throat, diarrhoea, headache, and reduced smell and taste. More severe cases can lead to loss/shortness of breath, chest pain, and potentially loss of speech or movement. It typically takes four to five days from infection until the symptoms show.
Publications
Humphrey et al. (2023): PROphylaxis for paTiEnts at risk of COVID-19 infecTion (PROTECT-V), Trials.
Jurgeit et al. (2012): Niclosamide Is a Proton Carrier and Targets Acidic Endosomes with Broad Antiviral Effects, PLOS Pathogens.
Weiss et al. (2020): Spatial and temporal dynamics of SARS-CoV-2 in COVID-19 patients: A systematic review and meta-analysis, EBioMedicine, a subjournal of The Lancet.
Jeon et al. (2020): Identification of Antiviral Drug Candidates against SARS-CoV-2 from FDA-Approved Drugs, ASM Journals
Backer et al. (2021): A randomized, double-blind, placebo-controlled phase 1 trial of inhaled and intranasal niclosamide: A broad spectrum antiviral candidate for treatment of COVID-19, The Lancet Regional Health Europe.
Weiss et al. (2021): Niclosamide shows strong antiviral activity in a human airway model of SARS-CoV-2 infection and a conserved potency against the Alpha (B.1.1.7), Beta (B.1.351) and Delta variant (B.1.617.2), PLOS One.
Braga et al. (2021): Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spike-induced syncytia, Nature
Singh et al. (2022): Niclosamide – a promising treatment for COVID-19, British Journal of Pharmacology.
Weiss et al. (2022): Topical niclosamide (ATx201) reduces Staphylococcus aureus colonization and increases Shannon diversity of the skin microbiome in atopic dermatitis patients in a randomized, double-blind, placebo-controlled Phase 2 trial, Clinical and Translational Medicine.
Cairns et al. (2022): Efficacy of Niclosamide vs Placebo in SARS-CoV-2 Respiratory Viral Clearance, Viral Shedding, and Duration of Symptoms Among Patients With Mild to Moderate COVID-19, JAMA Network (specifically kidney patients).
Weiss et al. (2022): Kidney Transplant and Dialysis Patients Remain at Increased Risk for Succumbing to COVID-19, Transplantation

