UNION was incorporated in 2011 as AntibioTx, initially focusing on infectious diseases and antimicrobial resistance (AMR).
In advanced screenings of well-characterized compounds, the company’s scientists identified several compounds with potential outside approved indications, including niclosamide.
During the work with the anti-bacterial properties of niclosamide, the team also discovered novel anti-inflammatory properties. This led to expansion of the disease focus to also include immunology with the starting point in immuno-dermatology. Because of the expanded therapeutic focus, the company was renamed to UNION therapeutics A/S in 2018.
In March 2020, researchers at the Pasteur Institute in Seoul screened a large number of molecules for activity against SARS-CoV-2. Niclosamide was identified to have a 40-fold higher potency in vitro against SARS-CoV-2 than remdesivir. UNION researchers managed to develop a high concentration solution of niclosamide. Niclosamide nasal spray was selected for the PROTECT-V platform study, led by Cambridge University Hospitals NHS Foundation Trust and the University of Cambridge, and funded by Kidney Research UK, LifeArc, Addenbrookes Charitable Trust and UNION. The platform trial was initiated in February 2021 investigating niclosamide nasal spray as a prophylaxis treatment in immunocompromised patients, who are at high risk of contracting COVID-19 due to their weak immune system due to medication or underlying diseases. These patients do not mount a proper antibody response due to vaccination and are thus often not sufficiently protected. The PROTECT-V study was granted Urgent Public Health (UPH) prioritization in 2021 by the National Institute for Health Research, and in March 2022, the niclosamide nasal spray arm of the PROTECT-V study was expanded to the George Institute for Global Health in India. Topline data from the niclosamide-arm of the study was reported in June 2023, thus unfortunately the study did not meet its primary endpoint of reduction in risk of infection. Read more about niclosamide.
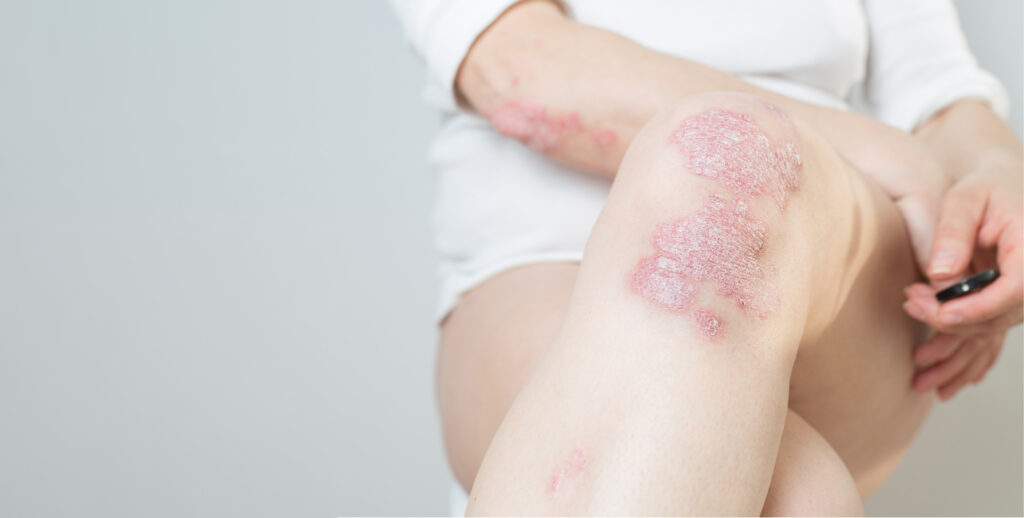
In June 2020, UNION acquired the full Phosphodiesterase 4 (PDE4) inhibitor program from LEO Pharma. The lead candidate, orismilast, has been selected as a next generation PDE4 inhibitor, with high selectivity for PDE4 subtypes particularly involved in immunologic disease. Orismilast is developed both as a tablet and as a topical cream. Positive results from the IASOS, Phase 2b study with orismilast for the treatment of psoriasis were reported in January 2023 supporting the target product profile of a best-in-class PDE4 inhibitor and confirming the well-established, favorable safety profile of PDE4 inhibition. In June 2023, positive topline results from the OSIRIS, investigator-initiated proof-of-concept study with oral orismilast in HS was reported. These results also confirm that orismilast is a safe and efficacious treatment.
Orismilast cream has been investigated in patients with mild-severe atopic dermatitis confirming a robust proof of concept. The broad immunomodulating effects of orismilast support the potential in other immunological diseases where unmet medical needs exist as well.