UNION is focused on clinical development of novel treatment options for patients with immunological diseases. Our core area of expertise is to identify the most suitable patient populations for our therapeutics programs based on a deep understanding of mode-of-action (MoA) and medical needs, and to rapidly develop them through clinical proof-of-concept (PoC), thereby creating significant value inflection points for the company.
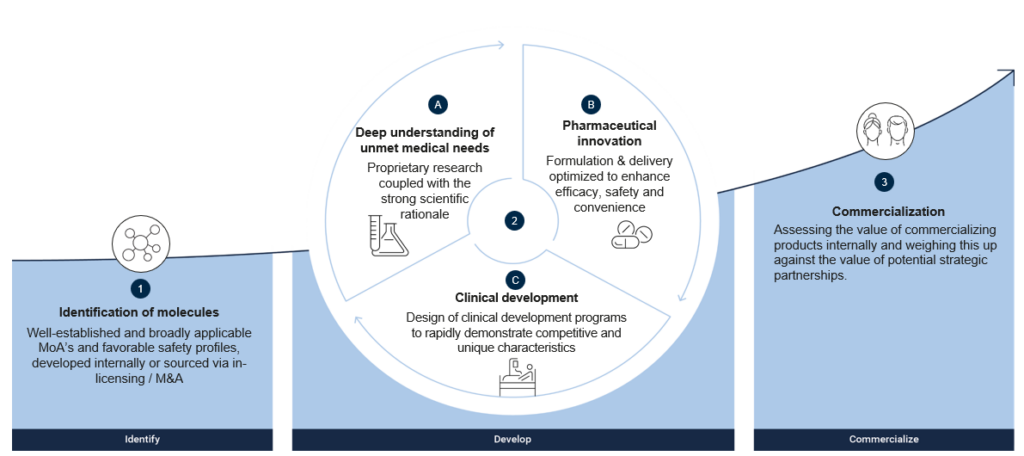
Identification of molecules
We work with molecules that have broadly applicable and well-understood MoA as well as having demonstrated safety. This allows us to focus on qualifying the relevance of the MoA’s for targeted diseases and effectively reducing the development risk compared to conventional discovery and development approaches. By focusing on a small number of molecules with broad applicability, we can effectively build deep molecule and MoA understanding and leverage preclinical, clinical, and regulatory work across indications.
Drug development
Once we have identified molecules for development, the development process begins. At UNION, we leverage deep expertise across four foundational pillars of pharmaceutical development in a holistic manner:
- Molecule and disease matching: We combine molecule and disease understanding to identify and prioritize diseases and patient populations where the MoA is most relevant and the unmet medical need is significant;
- Medical needs identification: We identify unmet needs for patients, physicians and payers, and develop solutions to meet the needs for all stakeholders;
- Pharmaceutical innovation: We optimize drug delivery to target specific tissues and to enhance efficacy, safety and ease-of-use of the drug to support patient adherence to treatment; and
- Clinical development: We utilize our expertise to develop and establish clinical development programs which are designed to rapidly demonstrate competitive and unique product characteristics for patients, physicians and payers. This includes early de-risking through innovative and efficient PoC studies with robust and predictive clinical endpoints.
Our development strategy enables potentially faster and lower-cost development of new pharmaceutical products while an established knowledge base relating to molecules and products in development can reduce overall risk and create new business opportunities.
Commercialization
To maximize the potential of the compounds and development programs, and the number of patients reached, collaboration with large pharma companies with existing commercialization capabilities is an integral part of UNION’s strategy. The nature and form of the collaboration, including the role of UNION, will vary depending on the size and characteristics of the indication, the stage of development as well as the geography.

